Understanding the Manufacturer's Warranty for Dental Implants
A Garantie für Implantate (warranty for implants) is a foundational component of modern implant dentistry, serving a dual purpose. For the manufacturer, it is a formal declaration of quality and confidence in their product's material science and engineering. For clinicians and distributors, it is a critical safety net that builds trust and mitigates financial risk in the rare event of a product defect. A clear understanding of what a manufacturer's warranty covers—and what it excludes—is essential for managing clinical expectations and professional liability.
Differentiating Product Warranty From Clinical Guarantee
In implant dentistry, it is crucial to distinguish between the product warranty issued by the manufacturer and the clinical guarantee provided by the dental professional. These two assurances cover distinct aspects of the treatment process and are not interchangeable.
- Product Warranty: This is the manufacturer's commitment, covering the physical integrity and performance of the implant and its original prosthetic components. It addresses potential defects in materials or workmanship.
- Clinical Guarantee: This is the practitioner's assurance, reflecting their professional skill in diagnosis, treatment planning, surgical execution, and post-operative care.
Clarifying this distinction with patients is a key part of the informed consent process, ensuring they understand the roles and responsibilities of all parties involved in their long-term care.
The Manufacturer’s Commitment: A Promise of Quality
A manufacturer's warranty is a legally binding assurance based on extensive research, development, and rigorous quality control. It is a testament to the product's reliability under specified clinical conditions.
Key points typically covered include:
- Product Replacement: If an implant fixture fractures under normal masticatory forces, the manufacturer will typically provide a replacement implant free of charge.
- Material Integrity: The warranty covers verifiable defects in the titanium alloy or issues with the surface treatment that could compromise osseointegration.
- Component Specificity: Validity of the warranty is almost always conditional upon the exclusive use of original, system-matched prosthetic components. Mixing and matching parts from different systems will void this protection.
This structured support allows clinicians to focus on delivering optimal patient care, confident that the foundational components are backed by the manufacturer.
The Clinician’s Role and Responsibility
The clinical guarantee is a reflection of the practitioner's expertise and adherence to established protocols. It encompasses everything from accurate diagnostics and precise surgical technique to patient management and follow-up care.
The success of implant therapy is a shared responsibility. Patients must understand that their compliance—particularly with oral hygiene protocols and recommended follow-up visits—is critical for ensuring the long-term success of the treatment. For a deeper analysis of this dynamic, explore our article on dental implant warranties.
A robust manufacturer warranty is more than a safety net; it is a statement of partnership. It signifies a shared commitment to achieving successful, long-term patient outcomes by ensuring the core components meet the highest quality standards.
Ultimately, transparent communication about both the product warranty and the clinical guarantee empowers patients, builds trust, and sets the stage for a successful clinical partnership.
Decoding Manufacturer vs Clinical Guarantees
In implant dentistry, the term "guarantee" requires precise definition. It is essential to differentiate between the manufacturer’s product warranty (Produktgarantie) and the clinician's treatment guarantee (Behandlungsgarantie). Conflating these two can lead to patient confusion and operational challenges for a dental practice.
The distinction is clear: the manufacturer guarantees the quality of the tool, while the clinician guarantees the expertise of its application. One covers the physical product; the other covers the clinical service and outcome.
The Manufacturer's Product Warranty Explained
At its core, a manufacturer's warranty is a formal promise regarding the physical integrity and performance of their products. It is a declaration that an implant and its associated components are free from defects in materials and workmanship. For clinicians and distributors, this serves as a vital assurance of the company's confidence in its engineering, quality control, and long-term clinical data.
This Garantie für Implantate is designed to cover specific, verifiable product failures, such as:
- Implant Fracture: The physical replacement of an implant body that fractures under normal occlusal loads.
- Material Defects: Verifiable flaws in the titanium alloy or surface treatment that prevent successful osseointegration.
- Component Integrity: Failure of original prosthetic components, such as abutments, due to a manufacturing defect.
A lifetime product warranty from a trusted manufacturer like Alfa Gate is not merely a policy; it's a statement of unwavering confidence in the product's design, material purity, and the scientific evidence supporting its long-term success in bone.
Equally important is understanding the exclusions. A product warranty is strictly limited to replacing the defective, company-manufactured product. It does not cover surgical fees, laboratory costs for the final restoration, or failures resulting from external factors. This is why adherence to a complete, original system, like the M+ Conical Connection implants, is critical—introducing third-party components will almost invariably void this protection.
The Clinician's Treatment Guarantee
While the manufacturer backs the product, the clinician backs the procedure. The clinical guarantee is a reflection of professional skill and the quality of care provided. This guarantee addresses the overall success of the treatment performed, not a physical defect in the implant itself.
The scope of a clinical guarantee is defined by the practice and is contingent upon factors under the clinician's direct control and assessment.
- Precise Case Selection: Successful outcomes begin with appropriate patient selection for implant therapy.
- Surgical Protocol: Adherence to established surgical and aseptic techniques is fundamental to predictable results.
- Patient Compliance: The patient's commitment to oral hygiene and attendance at follow-up appointments is a non-negotiable component of treatment success.
This guarantee is a professional promise. Clearly articulating its terms—including what steps will be taken if an implant fails to integrate or another complication arises—builds significant patient trust. It logically separates the product's performance from its skilled clinical application.
Manufacturer Warranty vs Clinical Guarantee At a Glance
| Feature | Manufacturer Warranty (Produktgarantie) | Clinical Guarantee (Behandlungsgarantie) |
|---|---|---|
| Who is Responsible? | The implant manufacturer (e.g., Alfa Gate). | The treating clinician or dental practice. |
| What is Covered? | The physical implant and original prosthetic components against defects. | The overall treatment outcome and clinical service. |
| Typical Coverage | Implant fracture, verifiable material flaws, component failure due to manufacturing error. | Failure of osseointegration, post-operative complications managed by the clinician. |
| Typical Exclusions | Surgical fees, lab costs, final restoration, issues from improper handling, or using non-original parts. | Failures due to poor patient hygiene, smoking, systemic conditions, trauma, or non-compliance with post-op instructions. |
| The Promise | "Our product is engineered to be free from defects." | "My clinical skill and care will facilitate a successful outcome." |
By clearly defining these two separate guarantees, you establish a transparent clinical environment where all parties understand their role in achieving a successful, durable outcome.
Ready to partner with a company that stands firmly behind its products? Become an Alfa Gate distributor and offer your clients the confidence that comes with a robust manufacturer warranty.
Getting to Grips with Common Warranty Exclusions
For effective risk management, understanding what a Garantie für Implantate excludes is as critical as knowing what it covers. A manufacturer's warranty is a promise of product integrity, conditional upon its proper use under appropriate medical circumstances.
Familiarity with common exclusions is key to setting clear patient expectations and ensuring a smooth claims process. These exclusions are not arbitrary; they are based on established clinical science and known risk factors that can interfere with osseointegration and long-term implant stability.
Systemic and Behavioural Factors
Certain patient-related factors can significantly compromise the biological processes required for healing and bone integration. Consequently, implant failures linked to these conditions are almost universally excluded from manufacturer warranties, as they represent external influences beyond the manufacturer's control.
Common patient-related exclusions include:
- Uncontrolled Systemic Conditions: Conditions such as unmanaged diabetes, severe osteoporosis, or therapies involving immunosuppressive drugs can severely impair the body's healing capacity.
- Heavy Smoking or Substance Abuse: Nicotine is a potent vasoconstrictor that restricts blood flow to the surgical site, depriving healing tissues of necessary oxygen and nutrients. This makes smoking a primary behavioural risk factor.
- Poor Oral Hygiene: Inadequate plaque control can lead to peri-implantitis, an inflammatory condition that causes progressive bone loss around the implant and can result in its failure.
- Trauma or Accident: Warranties cover failures under normal functional loads, not damage resulting from external forces such as a fall or sports-related injury.
Procedural and Component-Related Exclusions
The clinical application of the implant system is the other critical aspect. Warranty validity is contingent upon adherence to validated protocols. Deviations introduce variables the manufacturer cannot account for, thereby voiding the guarantee.
A manufacturer's warranty is built around a complete, validated system. Using non-original parts is analogous to using an unapproved engine oil in a new vehicle; it may appear to function, but it instantly voids the powertrain warranty because the manufacturer can no longer guarantee the system’s performance or integrity.
This is a critical consideration for any practice. While using third-party components may seem to offer short-term cost savings, it introduces significant clinical and financial risks by nullifying the Garantie für Implantate.
Key procedural exclusions to keep in mind:
- Use of Non-Original Components: The biomechanical precision of an implant system depends on the exact fit and material compatibility of the implant, abutment, and screw. Mixing components compromises this delicate balance, risking micromovement, screw loosening, or material fatigue. Systems like Alfa Gate’s Bioactive implants are engineered to function as a complete, integrated solution.
- Deviation from Surgical Protocol: Manufacturers provide detailed guidelines for drilling sequences, insertion torque, and healing periods based on extensive research. Ignoring these protocols—for example, by immediately loading an implant not designed for such an application—can lead to primary stability failure that is not covered.
- Improper Clinical Application: Placing an implant in a site with insufficient bone quality or quantity without appropriate bone regeneration is a contraindication. A failure in such a scenario is attributable to case selection, not a product defect.
A firm grasp of these exclusions allows for a more robust informed consent process. Clear communication about these boundaries protects the practice and reinforces the patient’s role as an active partner in their long-term oral health.
The Critical Role of Documentation and Traceability
In the context of a Garantie für Implantate claim, meticulous record-keeping is paramount. It is not merely administrative paperwork; it is a fundamental aspect of patient safety and the evidentiary basis for warranty validation. Without a robust documentation system, even a legitimate claim can face delays or rejection.
Each piece of information validates clinical decisions, confirms the use of authentic components, and tracks every item from the manufacturer to the patient. This chain of custody is a non-negotiable standard for medical devices.
Building a Bulletproof Documentation File
To ensure a smooth warranty claim process, documentation must be detailed and comprehensive. This professional diligence provides a clear record of treatment, leaving no room for ambiguity.
The clinical file must include:
- Lot and Serial Numbers: This is the foundation of traceability. Every implant and prosthetic component includes a sticker with unique identifiers that must be recorded in the patient's chart.
- Sterilisation Records: Logs of sterilisation cycles for all surgical instruments provide proof of adherence to aseptic protocols.
- Detailed Surgical Notes: These should include the date, implant site, implant dimensions (diameter and length), insertion torque values, and any relevant clinical observations made during the procedure.
- Radiographic Evidence: Pre-operative, immediate post-operative, and follow-up radiographs are essential for documenting bone levels and verifying implant position and stability over time.
- Informed Patient Consent: A signed consent form is vital, particularly one that outlines the patient's responsibilities regarding hygiene and follow-up care, as well as the warranty's limitations and exclusions.
The Power of Traceability
Traceability is a core principle of medical device regulation and is central to patient safety. It ensures that every single component can be tracked from its manufacturing batch to its final placement. For a Garantie für Implantate, this is how manufacturers confirm that genuine, system-matched parts were used.
Think of traceability as the product’s official passport. It documents its entire journey, confirming its origin, specifications, and authenticity. Without this verifiable record, a manufacturer has no way to confirm the integrity of the clinical assembly, a prerequisite for honouring a warranty.
This becomes especially critical if the use of third-party components is ever questioned. Detailed records proving the use of a complete, validated system—like Alfa Gate's M+ Conical Connection implants—are the strongest defense against a claim denial due to component mismatch. For further clinical insights, explore our related educational articles.
To build a practice founded on quality and trust, partner with a company that shares your commitment. Contact an Alfa Gate representative to discover how our products and support systems can benefit your clinic.
A Step-By-Step Guide To Filing a Warranty Claim
When an implant failure occurs, the claims process should be a clear, straightforward workflow designed for efficient resolution. Navigating a Garantie für Implantate claim begins long before the need arises, with meticulous day-to-day record-keeping.
This infographic highlights the foundational elements required for any successful warranty submission.
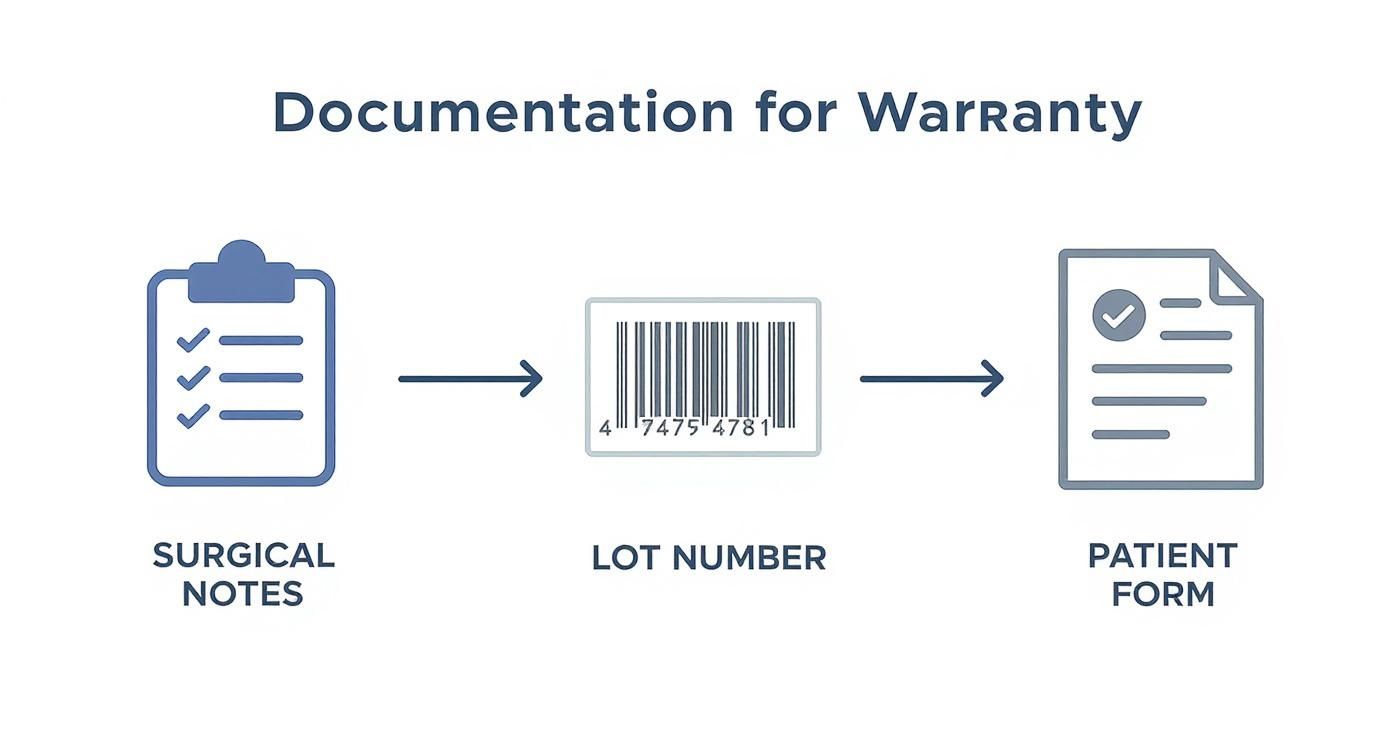
As illustrated, precise documentation is the backbone of a successful claim, from capturing the lot number to maintaining detailed clinical notes.
Kicking Off the Claim
Upon clinical confirmation of an implant failure, prompt notification to the manufacturer is the first step.
- Contact Your Representative: Your initial contact should be your local distributor or sales representative. They are trained to guide you through the process and provide the necessary forms.
- Provide Initial Details: Be prepared with the patient's anonymised case number, the implant type and dimensions, and the date of placement. This allows the support team to quickly access product records.
This proactive communication transforms a clinical challenge into a manageable administrative task.
Getting the Paperwork Right
Accuracy is essential when completing the manufacturer's claim form. This document serves as the official record of the event and is used for internal quality assurance investigations.
Treat the claim form as a clinical report, not just administrative paperwork. Detailed and precise information allows the quality assurance team to conduct a thorough analysis, which not only facilitates claim approval but also provides crucial data for post-market surveillance.
Ensure all sections are completed thoroughly, with special attention to clinical observations, radiographic evidence, and patient history. Your diligent documentation will make this information transfer seamless and accurate.
Handling and Returning the Device
The handling of the explanted device is a critical step. The manufacturer requires the physical implant to perform a root cause analysis.
- Sterilisation Protocol: Immediately after removal, sterilise the implant according to standard clinical protocols. Crucially, do not clean it with brushes or chemicals, as this can destroy surface evidence needed for analysis.
- Secure Packaging: Use the return kit provided by the manufacturer to ensure the device is transported safely and is correctly identified upon arrival.
- Include All Components: If possible, return all related components, such as the cover screw or healing abutment.
What to Expect During the Review
Once the claim form and explanted device are received, the manufacturer's technical team will conduct an analysis to identify any material or manufacturing defects. They will compare these findings with the clinical information you provided.
The review timeline can vary, but a well-documented and complete submission typically accelerates the process. For more information on navigating treatment costs, our detailed guide on German dental implant costs and insurance coverage offers valuable context.
Following these steps ensures an efficient and professional claims process, reinforcing the partnership between your practice and your implant provider. Should you have questions about a specific case, please contact an Alfa Gate professional for direct support.
How a Strong Warranty Shapes Your Implant Choice
When evaluating dental implant systems, clinical performance data is only part of the equation. A comprehensive manufacturer warranty is a powerful indicator of product reliability and long-term support. It signals the manufacturer's confidence in its own research, surface technology, and quality control processes.
For practice owners and distributors, the terms of a Garantie für Implantate are a key factor in risk assessment. A strong warranty significantly reduces financial exposure in the rare event of a product defect.
A Differentiator In A Competitive Market
In a crowded global market, warranty terms serve as a critical differentiator. A robust guarantee:
- Signals manufacturer confidence in its material science and engineering.
- Reduces the practice's financial and clinical risk profile.
- Enhances professional reputation by aligning the practice with a quality-focused partner.
In a mature and competitive market like Germany, which is projected to grow significantly, warranty coverage often becomes a deciding factor for clinicians and procurement managers.
A reliable warranty transforms the relationship from a simple transaction into a true clinical partnership. It demonstrates a shared responsibility for the patient’s long-term health and clinical success.
The Impact On Stocking And Treatment Decisions
For distributors and practice managers, warranty terms directly influence procurement and stocking decisions. A lifetime guarantee on an implant fixture, for instance, suggests a product engineered for longevity and durability. This translates into:
- Greater confidence in inventory management.
- Increased assurance during treatment planning and execution.
- A clear benchmark for comparing suppliers and systems.
When a clinical team selects an implant system backed by a robust guarantee, they can focus entirely on achieving excellent clinical outcomes rather than questioning component quality. For a closer look at what defines the best dental implants in Germany, our guide offers further insights.
Ultimately, a strong warranty is a quality filter. It guides clinicians and distributors toward implant systems that deliver superior clinical performance, protect the practice's financial health, and foster lasting patient trust.
Explore implant systems that offer both clinical excellence and robust warranty support. Contact Alfa Gate to learn how our products and partnership can benefit your practice.
Answering Your Top Questions on Implant Warranties
Clinicians and distributors frequently ask for clarification on the finer points of implant warranties. Here are answers to some of the most common questions.
Does a Lifetime Implant Warranty Cover the Crown or Bridge, Too?
Typically, no. A manufacturer’s lifetime Garantie für Implantate is specific to the implant fixture and the original, system-matched prosthetic components (e.g., abutments).
The final restoration—the crown, bridge, or denture—is a custom-made device fabricated by a dental laboratory. It is not part of the implant manufacturer's product line. The dental practice or the laboratory usually provides a separate, shorter-term guarantee on this prosthetic work.
What Happens if a Patient Voids Their Warranty?
If a patient's actions, such as resuming smoking or neglecting oral hygiene, lead to implant failure, they have voided the terms of the warranty. In this case, the manufacturer is no longer obligated to provide a replacement product.
The clinical and financial responsibility for remediation then rests with the clinician and the patient. This scenario underscores the critical importance of a thorough informed consent process and detailed documentation of all patient instructions and compliance records.
Understanding the scope of a warranty is fundamental. Using third-party components will void most manufacturer warranties because the original manufacturer cannot guarantee the fit, performance, or biomechanical integrity of parts they did not design or test.
Adhering to an integrated system from a single manufacturer ensures that all components are designed for optimal compatibility and performance. This practice keeps the warranty fully intact, protecting the patient, the clinician, and the practice.
To ensure predictable outcomes with a system backed by a robust warranty, explore the Alfa Gate M+ Conical Connection implants. For further questions or to discuss partnership opportunities, we encourage you to contact us directly.







